Report Overview
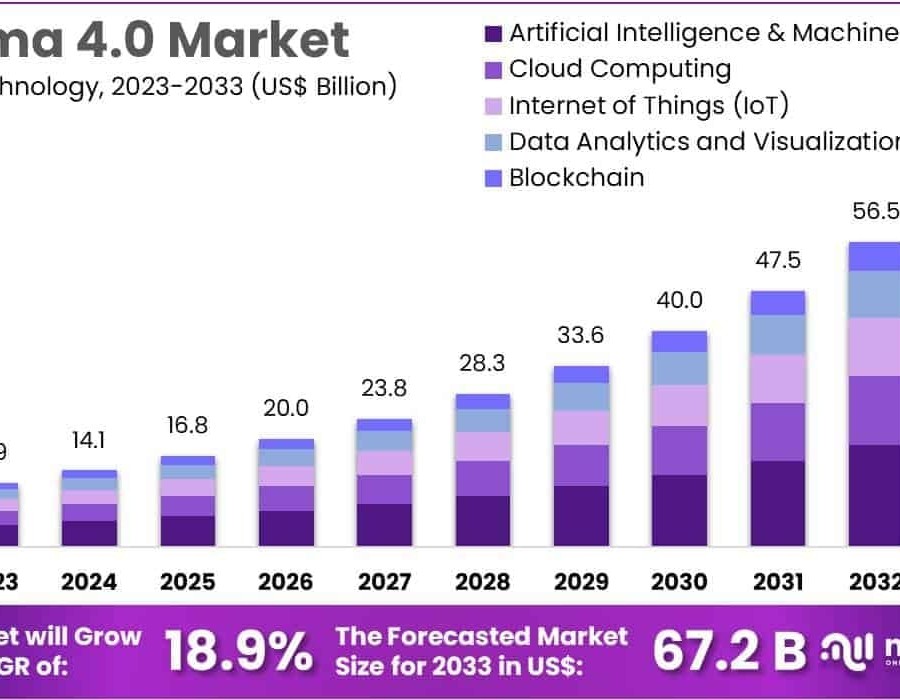
The Global Pharma 4.0 Market size is expected to be worth around US$ 67.7 Billion by 2033, from US$ 11.9 Billion in 2023, growing at a CAGR of 18.9% during the forecast period from 2024 to 2033.
The Pharma 4.0 Market is entering a transformative phase in 2025, driven by increasing adoption of AI-powered automation across pharmaceutical manufacturing. The integration of real-time data analytics, predictive maintenance, and robotic process automation (RPA) is streamlining production and reducing errors. Pharma companies are transitioning from batch-based to continuous manufacturing models, enabled by smart sensors and cloud platforms.
Regulatory support for digitized quality control and the need for faster time-to-market for novel drugs are accelerating adoption. Startups and tech firms are increasingly collaborating with legacy pharmaceutical companies to deploy modular, scalable digital systems. With a focus on lowering operational costs and improving compliance, Pharma 4.0 is not just a concept—it’s becoming the digital backbone of pharma operations.
Click here for more information: https://market.us/report/pharma-4-0-market/

Key Market Segments
By Technology
- Cloud Computing
- Internet of Things
- Artificial Intelligence & Machine Learning
- Data Analytics
- Visualization
- Blockchain
By Application
- Drug Discovery & Development
- Clinical Trials Management
- Manufacturing & Supply Chain Management
- Pharmacovigilance & Safety Monitoring
- Personalized & Precision Medicine
By End-user
- Pharmaceutical Companies
- Biotechnology Companies
- Contract Research Organizations
- Healthcare Providers
- Regulatory Authorities
Market Key Players
- Care
- Siemens Healthcare GmbH
- Oracle Corporation
- Microsoft Corporation
- Lupin
- IBM Corporation
- GE Healthcare
- Cisco Systems, Inc.
- ABB
Get a Sample Copy of the Report to Know More: https://market.us/report/pharma-4-0-market/request-sample/
Emerging Trends
A key trend is the shift to continuous manufacturing using AI-driven quality control systems. This transition improves real-time product release, reduces waste, and enhances productivity. AI models now predict deviations before they occur, transforming the traditional reactive quality assurance model.
Use Cases
A global pharmaceutical firm implemented AI-based monitoring in their vaccine production line. The system detected process deviations in real time and automatically adjusted parameters to maintain compliance, reducing production downtime by 30%. This case highlights smart compliance and operational continuity.
Contact us on
Market.us (Powered By Prudour Pvt. Ltd.)
Email: [email protected]
Address: 420 Lexington Avenue, Suite 300,
New York City, NY 10170, United States
Tel: +1 718 618 4351




Comments