In recent years, CAR T-Cell Therapy in India has emerged as a game-changer in the fight against certain aggressive blood cancers like leukemia, lymphoma, and multiple myeloma. This personalized immunotherapy treatment uses the power of genetically modified T-cells—specialized white blood cells—to target and eliminate cancer cells in the body.
With growing clinical success, India is quickly becoming a promising destination for patients worldwide seeking effective, affordable, and cutting-edge cancer treatment options. Let’s delve deeper into what CAR T-cell therapy involves, who it’s for, and why India is increasingly preferred for this revolutionary treatment.
What is CAR T-Cell Therapy?
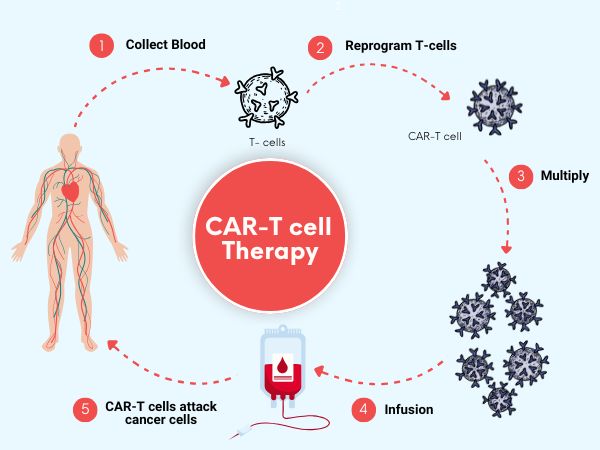
CAR (Chimeric Antigen Receptor) T-cell therapy is an advanced immunotherapy that reprograms a patient’s own T-cells to identify and attack cancer cells. These cells are collected through a process called leukapheresis, genetically engineered in specialized labs, and then infused back into the patient to fight cancer more effectively.
This therapy is primarily used for treating:
- B-cell acute lymphoblastic leukemia (ALL) in children and young adults
- Relapsed or refractory Non-Hodgkin Lymphoma (such as Diffuse Large B-cell Lymphoma)
- Multiple Myeloma that no longer responds to standard therapies
Who Needs CAR T-Cell Therapy?
CAR T-cell therapy is typically considered when:
- Standard treatments have failed (relapsed or refractory cases)
- The cancer is aggressive and fast-progressing
- The patient is physically capable of undergoing a highly personalized and intensive treatment plan
This therapy is not suitable for everyone. Pre-treatment evaluations check organ functions (heart, liver, kidneys), cancer stage, and infection status before moving forward.
Types of CAR T-Cell Therapies Available
India offers several types of CAR T-cell therapies approved globally and within clinical trials:
- ABECMA – For adults with relapsed multiple myeloma
- BREYANZI – Targets large B-cell lymphoma and follicular lymphoma
- CARVYKTI – Treats refractory multiple myeloma
- KYMRIAH – Effective for diffuse large B-cell lymphoma and B-cell ALL
India is also leading research on NexCAR19 (developed by IIT Bombay, Tata Memorial Centre, and ImmunoACT) and Varnimcabtagene autoleucel (by Immuneel Therapeutics). These Indian-developed therapies have shown impressive success rates in clinical trials—up to 83.3% response in lymphoma patients.
The Procedure: A Multi-Stage Process
1. Pre-Therapy Evaluation
Comprehensive lab tests and imaging (MRI, PET-CT, bone marrow biopsy) confirm cancer type, stage, and patient eligibility.
2. Leukapheresis
T-cells are collected from the patient’s blood using a specialized filtration technique.
3. T-cell Engineering
In certified labs, T-cells are genetically modified to express cancer-fighting receptors—a process that takes 2–5 weeks.
4. Infusion and Monitoring
Once engineered, the T-cells are reintroduced into the patient’s body. Hospitalization for 1–2 weeks is typical to monitor side effects.
Risks and Complications
Although highly effective, CAR T-cell therapy can cause serious side effects:
- Cytokine Release Syndrome (CRS): Inflammatory response with symptoms like high fever, low blood pressure, or organ dysfunction.
- Neurotoxicity: Can lead to confusion, seizures, or even coma.
- Infections: Weakened immune response increases risk for bacterial, viral, or fungal infections.
- Psychological Stress: Anxiety, PTSD, or cognitive changes may occur post-treatment.
That’s why CAR T-cell therapy is provided in specialized, well-equipped centers capable of handling complex side effects.
Recovery and Long-Term Care
Recovery requires regular follow-up visits to monitor blood counts, organ function, and detect signs of relapse.
Long-term care includes:
- Psychological counseling
- Immune system rebuilding
- Re-vaccination planning
- Fertility and endocrine system assessments
These ensure a holistic recovery for patients undergoing this intense yet promising treatment.
Success Rate of CAR T-Cell Therapy in India
According to a report published in The Lancet Haematology, CAR T-Cell Therapy in India has shown a success rate of 73% in patients with B-cell leukemia and lymphoma. Some Indian clinical trials, like those of NexCAR19 and Immuneel’s therapies, even reported response rates above 80%.
This highlights India’s growing potential as a leader in advanced cancer treatment through homegrown CAR T-cell therapies.
Cost of CAR T-Cell Therapy in India
One of the major advantages of seeking CAR T-Cell Therapy in India is affordability. While similar treatments in the US or Europe may cost over $400,000, the cost of CAR T-cell therapy in India ranges from $100,000 to $110,000.
This includes:
- Diagnostics and lab work
- Cell collection and modification
- Hospitalization and infusion
- Monitoring and follow-up care
With world-class facilities, India provides cutting-edge treatment at a fraction of the international cost.
Why Choose India for CAR T-Cell Therapy?
- High Success Rates: Indian therapies like NexCAR19 show strong clinical results.
- Affordable Treatment: Competitive pricing makes India accessible to global patients.
- Skilled Specialists: Oncologists and hematologists in India are well-trained and experienced.
- World-Class Infrastructure: Certified centers and labs are equipped with the latest technologies.
- Medical Tourism Support: From visa assistance to travel and stay—India offers holistic care.
Conclusion
CAR T-Cell Therapy in India is a beacon of hope for cancer patients who have exhausted traditional treatment options. With remarkable clinical success, affordable pricing, and advanced medical infrastructure, India stands at the forefront of cancer immunotherapy.
Whether you are a patient or caregiver, exploring CAR T-cell therapy in India could be the life-changing step toward a cancer-free future.




Comments