Introduction
In the competitive world of modern manufacturing, healthcare, and life sciences, maintaining product quality and regulatory compliance is more important than ever. Organizations face challenges in identifying, documenting, and preventing recurring quality issues. That’s where an Enterprise CAPA Management System becomes essential.
Qualityze offers a comprehensive, cloud-based Enterprise CAPA Management System that helps organizations streamline their Corrective and Preventive Actions (CAPA), strengthen compliance, and ensure continuous quality improvement across all departments and locations.
What is an Enterprise CAPA Management System?
An Enterprise CAPA Management System is a centralized solution that automates the entire CAPA lifecycle — from identifying nonconformances and conducting root cause analysis to implementing corrective and preventive actions.
It enables organizations to:
- Detect quality issues proactively
- Eliminate recurring problems
- Maintain traceable records for regulatory audits
- Integrate CAPA with other Quality Management System (QMS) modules
By using an enterprise-level solution like Qualityze EQMS Software, businesses can ensure standardization, transparency, and accountability across the organization.
Why Choose Qualityze Enterprise CAPA Management System
Qualityze provides a cloud-powered, enterprise-grade CAPA management system built to align with global regulatory frameworks such as ISO 9001, ISO 13485, GMP, and FDA 21 CFR Part 11. Here’s what sets it apart:
- Centralized CAPA Control: Manage all CAPA activities across departments from one unified platform.
- Configurable Workflows: Adapt CAPA processes to your organization’s structure and regulatory needs.
- Cloud-Based Accessibility: Access your CAPA data securely anytime, anywhere.
- Integrated Modules: Connect seamlessly with Nonconformance, Audit, Risk, and Document Management Systems.
- Audit-Ready Documentation: Maintain complete traceability for inspections and certifications.
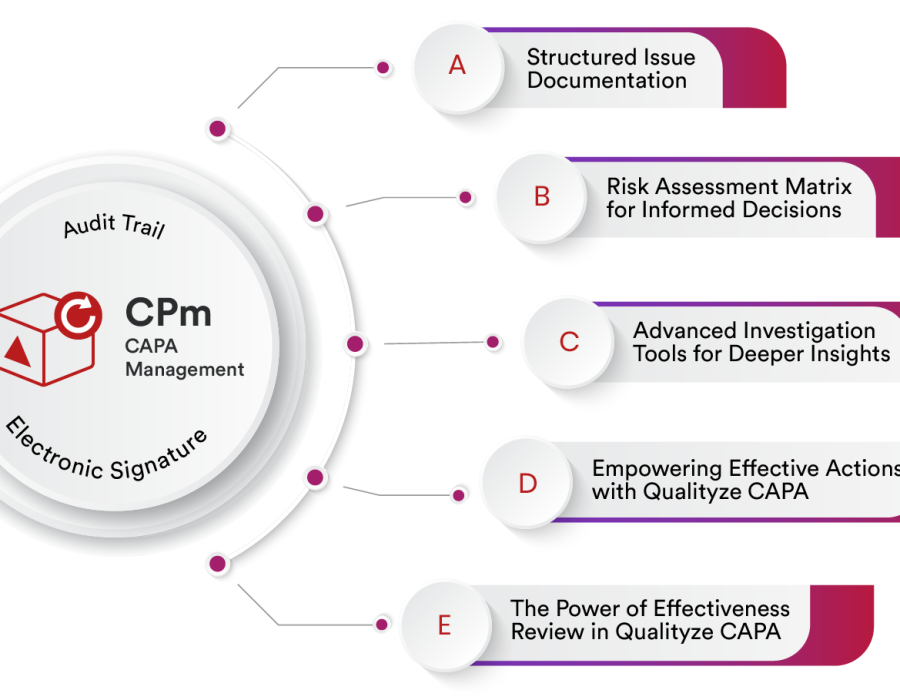
Key Features of Qualityze CAPA Management System
- Automated CAPA Workflows: From initiation to closure, every step is tracked and documented.
- Root Cause Analysis (RCA): Identify underlying issues with structured investigation tools.
- Real-Time Reporting: Monitor CAPA progress and effectiveness through live dashboards.
- Electronic Signatures: Ensure authenticity and compliance with digital records.
- Notifications & Alerts: Stay updated on pending actions and approvals.
- Compliance Templates: Simplify documentation aligned with regulatory standards.
Benefits of Implementing Qualityze Enterprise CAPA System
Improved Compliance: Meet ISO and FDA requirements effortlessly.
Faster Issue Resolution: Reduce turnaround time for CAPA closure.
Enhanced Collaboration: Empower cross-functional teams with real-time access.
Risk Reduction: Prevent recurrence of issues with effective preventive actions.
Continuous Improvement: Build a culture of quality and proactive problem-solving.
Industries Using Qualityze Enterprise CAPA Management System
- Pharmaceuticals & Life Sciences: Ensure FDA and GMP compliance with automated CAPA tracking.
- Healthcare: Strengthen patient safety and operational quality.
- Manufacturing: Minimize defects and improve product reliability.
- Automotive: Enhance supplier quality and production standards.
- Food & Beverage: Maintain compliance and prevent costly recalls.
How Qualityze Simplifies CAPA for Enterprises
Qualityze enables enterprises to transform their CAPA management process from reactive to proactive. The system integrates seamlessly with other quality management modules to create a connected quality ecosystem.
With real-time analytics, configurable workflows, and cloud accessibility, Qualityze ensures that CAPA is not just a compliance requirement but a strategic driver for continuous improvement.
Conclusion
An Enterprise CAPA Management System is no longer a luxury—it’s a necessity for achieving regulatory compliance, operational efficiency, and long-term quality excellence.
Qualityze CAPA Management System empowers organizations to standardize processes, enhance collaboration, and ensure continuous improvement across all levels of the enterprise.
By automating and simplifying CAPA workflows, Qualityze helps your business build a culture of quality, reduce risks, and achieve sustainable success.




Comments